Hip Resurfacing: Factors Influencing Survival
- Products
- ADEPT® Hip Resurfacing System
- Factors Influencing Survival
Appropriate device design is an essential factor for the performance of a hip resurfacing device. Different metallurgy, clearance, geometry and fixation are proven to affect clinical outcome and, consequently, results have differed greatly between devices (Figure 6a).
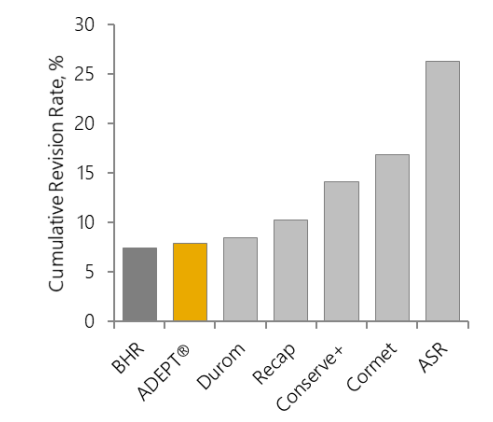
Figure 6: 10-year cumulative revision rates for brands of hip resurfacing device: a. all resurfacing brands in the NJR5; b. the ADEPT® and BHR in the NJR5 (no significant difference); c. the ADEPT® and BHR in the NJRR19 (the NJRR now only reports on devices currently in use in Australia).
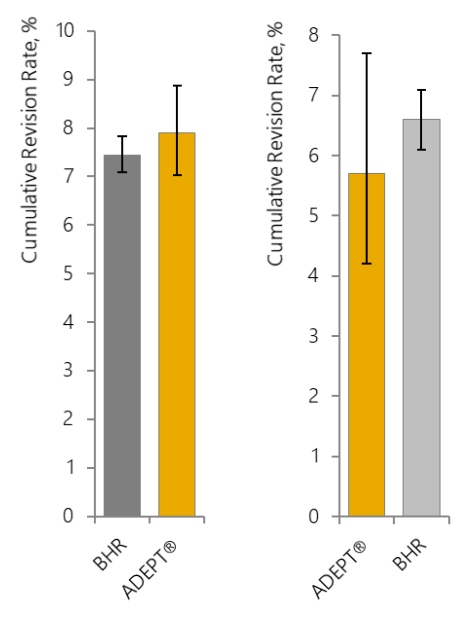
Of the devices shown in Figure 6a, only the ADEPT® and BHR are the original ‘modern-day’ hip resurfacing implants with the same clearance, metallurgy, geometry and fixation based on analysis of the successful early MoM devices and both manufactured by Finsbury (now MatOrtho®; BHR manufactured from start until 2009). Now only the ADEPT® is provided by the original manufacturers (MatOrtho®).
Success of MoM hip resurfacing is brand specific
The early clinical success of the concept led to the release of several other devices into the market. Most are no longer available due to poor design and manufacturing tolerances, rapid release to the market without adequate training to surgeons and overall poor clinical outcomes5,19. Therefore, revision rates vary widely by brand, with the best performing devices (ADEPT® and BHR) having 10 years cumulative revision <8% and the worst performing brand >29%.
National registry data (Figure 6a) includes all time use of resurfacing, which includes the smallest head sizes now withdrawn from all brand ranges (due to poorer performance than larger head sizes). The data also captures the wider patient selection indicated in early use of hip resurfacing (which were initially offered to all-comers similar to a THR population) and the acknowledged learning curve27,28 related to the introduction of the hip resurfacing concept (which is a fundamentally different technology to THR). Therefore, national joint registry data represents a ‘worst’ case in terms of survival analysis of hip resurfacing devices. Nonetheless, ADEPT® cumulative revision rate ranges from 2.3% to 11.1% at 10 years, which is comparable to the range recorded for total hip (2.42% to 12.6% at 10 years)5,19,25,29. Closer representation of current use includes revision rates of 2.3% for individual surgeon males only43 to 9.6% for all-time NJR use of the current size range31 at 15 years post-operation.
Probability estimations for survivorship of ADEPT® Hip Resurfacing are improving over time as the cohorts of patients on whom the estimations are based mature – the more patients with hips ‘at risk’ at longer time periods, the more accurate the estimation becomes. Figure 7 shows the difference in estimated probability of survivorship in May 2014, based on a starting cohort of over 3,000 patients recorded in the NJR from 2004 onwards29, compared to the estimated probability of survivorship in February 202230. The difference is simply that in early estimates few patients had reached >5 years post operation.
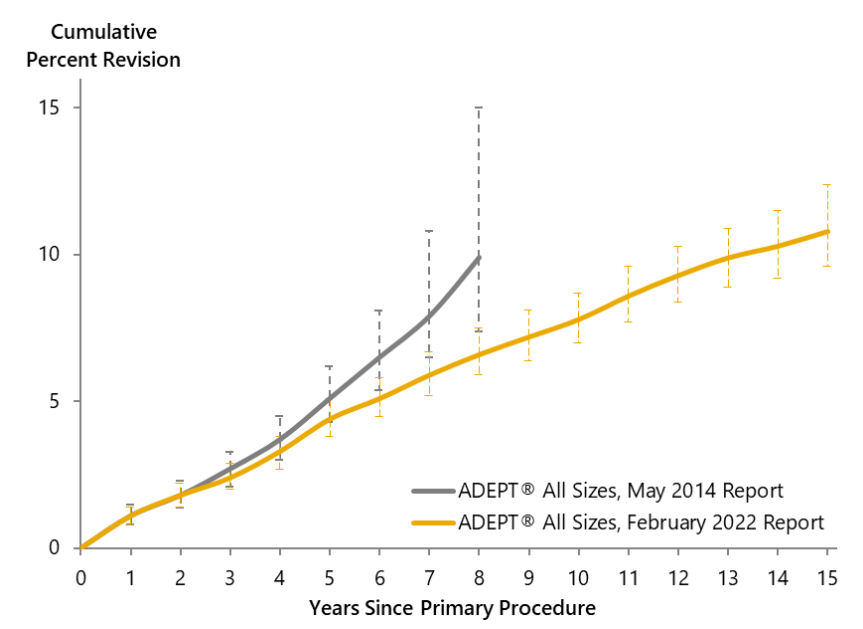
Figure 7: Cumulative percent revision rate of the ADEPT®, including all bearing sizes 38-58mm, males and females, for essentially the same patient cohort, reported in May 2014 (3,221 patients from 2004 to December 2013)29 and February 2022 (3,949 hips from 2004 to December 2021)30.
When first used, the ADEPT® Hip Resurfacing was available with femoral head diameters 38mm to 58mm. In 2013, the available data indicated that the revision rates for smaller head sizes (<46mm) were trending above 1% per year. Based on this data MatOrtho® voluntarily withdrew the smaller bearings (38-44mm) from the market. National Registry data does not accurately reflect current use of ADEPT®: registry data presents revision rates for all-time use, including all sizes, both genders, with the initial wider patient selection criteria, and learning curve for many surgeons. However, a review of clinical outcomes for the last 10 years only (46-58mm heads) demonstrates a more relevant cumulative revision rate, which is 4.5% (95% CI 3.3 to 6.0) at 10 years31. This rate is statistically equivalent to cemented and cementless THRs in younger age groups5.
For all ADEPT® implanted within the last 10 years (46-58mm head sizes), the 10-year revision rate is 4.5% (95% CI 3.3 to 6.0).
Based on the growing evidence for its success in well-selected patients and the ongoing support for the concept from the device manufacturer, use of the ADEPT® has grown in recent years32. This is evident in the NJR5 and NJRR19. The ADEPT® Hip Resurfacing is now the most-used resurfacing device in Australia, accounting for over 56% of all hip resurfacings in the last reported year19.
References
5. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 18th Annual Report, 2021: Surgical data to 31 December 2020. www.njrreports.org.uk.
6. Barrack RL, Ruh EL, Berend ME, Della Valle CJ, Engh A Jr, Parvizi J, Clohisy JC, Nunley RM. Do Young, Active Patients Perceive Advantages After Surface Replacement Compared to Cementless Total Hip Arthroplasty? Clin Orthop Relat Res. 2013; 471: 3803–3813
19. Australian Orthopaedic Association National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report. Adelaide: AOA. 2021. https://aoanjrr.sahmri.com.
24. The Swedish Hip Arthroplasty Register. Annual Report 2015. https://registercentrum.blob.core.windows.net/shpr/r/Annual-Report-2015-H19dFINOW.pdf. Accessed 6 Dec 18.
25 Van der Straeten C. Independent Post Market Surveillance Report on ADEPT (worldwide data sources). Internal report reference ER-2020-011.
26. Australian Orthopaedic Association National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report. Adelaide: AOA. 2020. https://aoanjrr.sahmri.com.
27. Gross TP, Liu F, Webb LA. Clinical outcome of the metal-on-metal hybrid Corin Cormet 2000 hip resurfacing system: an up to 11-year follow-up study. The Journal of arthroplasty. 2012; 27(4):533-8.
28. Mont MA, Schmalzried TP. Modern metal-on-metal hip resurfacing: important observations from the first ten years. JBJS. 2008; 90(Supplement_3):3-11.
29. Northgate Information Solutions Ltd. NJR Implant Summary Report for the ADEPT® Hip Resurfacing. May 2014. Ref: IQMSummary.Adept Resurfacing Head.15/05/2014.
30. NEC Software Solutions (UK) Limited. NJR Implant Summary Report for ADEPT® Hip Resurfacing. February 2022. Ref: Summary.Report.HP_Head_Adept Resurfacing Head_All.18/02/2022.
31. NEC Software Solutions (UK) Limited. NJR Implant Summary Report for ADEPT® Hip Resurfacing. February 2022. Ref: Summary.Report.HP_Head_Adept Resurfacing Head (Sizes 46-58 only)_All.18/02/2022.
32. MatOrtho internal data, February 2022.
33. Holland JP, Langton DJ, Hashmi M. Ten-year clinical, radiological and metal ion analysis of the Birmingham Hip Resurfacing: from a single, non-designer surgeon. JBJS- Br, 2012. 94(4):471.
34. Hing CB, Back DL, Bailey M, Young DA, Dalziel RE, Shimmin AJ. The results of primary Birmingham hip resurfacings at a mean of five years: An independent prospective review of the first 230 hips. J Bone Joint Surg (Br). 2007; 89-B:1431-1438.
35. Khan M, Kuiper JH, Edwards D, Robinson E, Richardson JB. Birmingham Hip arthroplasty five to eight years of prospective multicenter results. J Arthroplasty, 2008. Article in press. 36 Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg (Br). 2006; 88-B: 592-600.
37. Vendittoli PA, Lavigne M, Roy AG, Lusignan D. A prospective randomized clinical trial comparing MoM total hip arthroplasty and MoM total hip resurfacing in patients less than 65 years old. Hip Intl. 2006; 16 (Suppl 4): 73-81.
38. Plant JGA, Prosser GH, Burston BJ, Edmondston SJ, Yates PJ. Mid-Term Review of ADEPT Metal-On-Metal Hip Prosthesis. Functional, Radiological and Metal Ion Analysis. Open Journal of Orthopaedics, 2014, 4: 38-43.
43. Gani MH, Zahoor U, Hanna SA, Scott G. Metal-On-Metal hip resurfacing arthroplasty provides excellent long-term survivorship and function in patients with a good-sized femoral head. Results of a single, non-designer surgeon’s cohort. Bone & Joint Open. 2022; 3(1): 68-76.
Resources
ADEPT® Clinical Rationale
ADEPT® Flyer
ADEPT® Operative Technique
Download Now
Fill in your details below to download the ADEPT® Clinical Rationale
Additional Text
Download Now
Fill in your details below to download the ADEPT® Flyer.
Additional Text
Download Now
Fill in your details below to download the ADEPT® Operative Technique
PROMs Data
The mean postoperative hip score for hip resurfacing is reported to be in the ‘excellent’ category in numerous published studies4,6,33,34,35.
Based on up-to-date systematic review of peer-reviewed literature published in 2019, MoM hip resurfacing is associated with superior outcomes when compared to THR4. The review includes findings such as decreased thigh pain6, less limp with walking6, improved function14, superior UCLA activity scores36, 37, quality of life36,37 and return to manual labour work36, moderate/heavy activity37, sport36 and long distance walking and running6.