Patient Area
- Home
Patient Information
Patients have and will always be the top priority at MatOrtho. We believe that patients have a right to research and find out more about the procedure they’ll receive. To help provide more information and confidence from a patient perspective we have created a dedicated area with inspiring stories and testimonials.
Following a joint replacement, it’s vital that patients get back to an active, everyday life. MatOrtho orthopaedic products have a clinically proven track record of patient satisfaction, resulting in fewer hip and knee postoperative complications.


Hip Replacement Information
Hip replacement, also known as hip arthroplasty, is a surgical procedure in which a damaged hip joint is replaced with an artificial joint. This procedure is typically performed to relieve pain and improve function in individuals with hip joint damage. The artificial joint, or prosthesis, may be made of metal, plastic, or ceramic materials and is designed to mimic the movement and stability of a healthy hip joint. The benefits can include reduced pain, improved mobility, and increased independence.
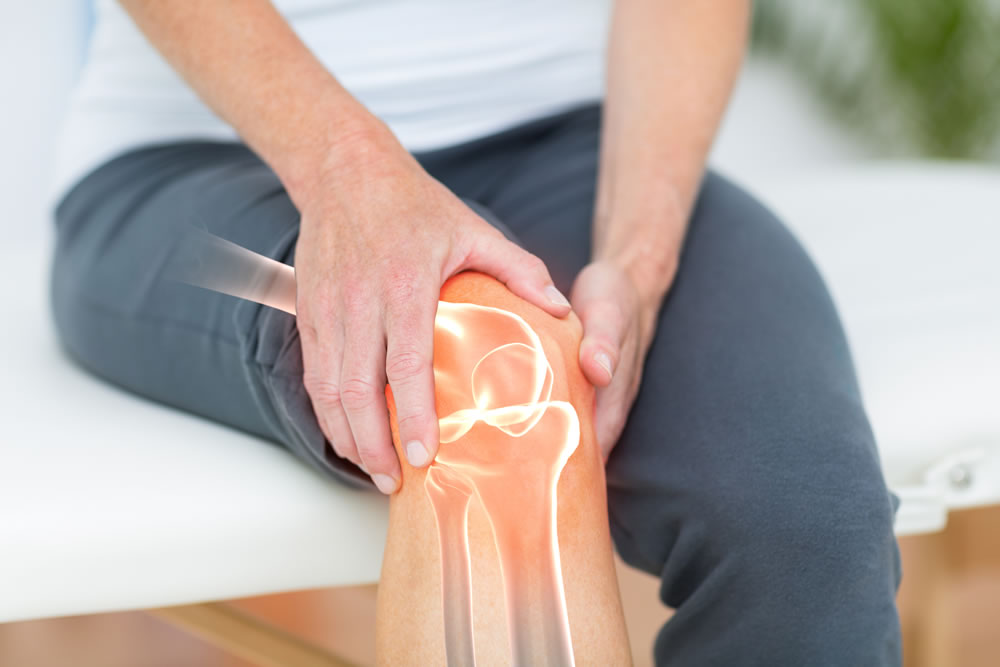
Knee Replacement Information
Knee replacement, also known as knee arthroplasty, is a surgical procedure in which the damaged parts of a knee joint are removed and replaced with artificial parts (prosthetics). This procedure is typically performed to relieve pain and improve the function of a damaged knee joint. During surgery, the damaged bones and cartilage are removed and replaced with metal and plastic parts. The benefits of knee replacement surgery can include reduced pain, improved mobility, and increased independence.

Patient Testimonials
Several of the patients who have received one of our orthopaedic devices have been kind enough to share their truly inspiring stories with us. The decades of study and clinical research that went into developing these implants, paired with the skill of experienced surgeons, have permitted these individuals to resume normal active lives – and more. However, it's important to keep in mind that patient testimonials are subjective experiences and may not be representative of everyone's experience.
.png)
